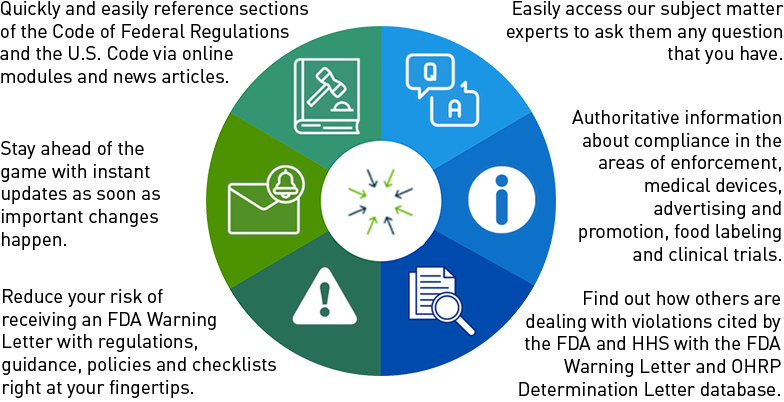
New FDA Draft Guidance Focuses on When and How To Use Data Monitoring Committees
February 16, 2024 at 03:21 PM EST
In a new draft guidance released Feb. 12, the FDA “strongly recommends” using a data monitoring committee (DMC) if subjects in a clinical trial “are at risk of serious morbidity or mortality.”
The FDA issued the new guidance because “there has been an increase in the use of DMCs in many disease areas” since the FDA released guidance in 2006.
The agency noted that DMCs also c... Read More